The Rapid Response Vaccine Development Project
Quanticate partnered with a top global pharmaceutical company to deliver essential programming support, helping to fast-track the development of a COVID-19 vaccine for children through agile collaboration, 24/7 global coverage, and rigorous data accuracy, playing a crucial role in the sponsor securing FDA emergency use authorisation.
Case Study Introduction
The COVID-19 pandemic posed unprecedented challenges to the global healthcare system, creating an urgent need for effective vaccines.
A number of pharmaceutical companies urgently undertook the critical task of developing and testing a COVID-19 vaccine under extreme time constraints and high stakes. Our top 5 pharma sponsor played an extensive role in meeting this challenge undertaking several rapid response studies. Quanticate played a pivotal role in supporting the sponsor’s programming needs, contributing to the successful and timely completion of this essential project.
Sponsor Overview and Pandemic Response
The sponsor is one of the world’s leading biopharmaceutical companies working under difficult conditions in pushing its extensive work program to develop and deliver vaccines to combat the COVID-19 pandemic. Not surprisingly, their studies were essential and crucial to this effort, requiring completion under extremely tight timelines due to the urgency of the pandemic and similar efforts from their direct competitors.
To overcome these challenges, the sponsor leveraged their long-standing partnership with Quanticate to provide expert programming support, ensuring that all data met the rigorous standards necessary for timely regulatory approval.
Objectives and Key Challenges
The sponsor is one of the world’s leading biopharmaceutical companies working under difficult conditions in pushing its extensive work program to develop and deliver vaccines to combat the COVID-19 pandemic. Not surprisingly, their studies were essential and crucial to this effort, requiring completion under extremely tight timelines due to the urgency of the pandemic and similar efforts from their direct competitors. To overcome these challenges, the sponsor leveraged their long-standing partnership with Quanticate to provide expert programming support, ensuring that all data met the rigorous standards necessary for timely regulatory approval.
Facing tight regulatory deadlines? Discover how our programming specialists can accelerate your project timeline. Contact us today for a custom solution.
Strategic Approach and Tailored Solutions
Quanticate employed a highly collaborative and agile approach to support our clients’ studies. Our team provided continuous programming support, often working extended hours and weekends to meet tight deadlines. By combining our own suite of internal tools and methodologies with our deep expertise in the sponsor’s internal systems, we implemented an optimal solution to ensure data integrity and efficiency. This enabled the rapid analysis and reporting of study data. Despite the intense pressure, the team maintained high-quality standards, which were critical to the study’s success.
Measurable Impact and Outcomes
Quanticate’s contribution was instrumental in achieving several key milestones for the sponsor during 2022. Our efforts ensured the timely submission of data to regulatory bodies, culminating in the FDA's emergency use authorisation of the sponsors COVID-19 vaccine for children, including those as young as 6 months old.
The success of this vaccine led to a significant increase in market share and revenue, which not only solidified their market dominance, but also increased their influence on global health policies and had a profound impact on public health, providing a safe and effective vaccine for younger populations.
Sponsor Feedback and Testimonials
The Sponsor’s feedback highlighted Quanticate’s exceptional work on the COVID studies. Ready for a partner you can trust? Let us show you how Quanticate can enhance your team’s capabilities. Request a consultation today.
“I would like to recognize your outstanding work and to express my sincere gratitude. You have displayed the following characteristics: Customer Relationship; Teamwork and Collaboration. Thank you for your contribution to our deliverables."
"You have displayed admirable characteristics which lead to a more trusting and effective partnership between Quanticate and our company. As we work together effectively, we are showing great benefit to our studies and most importantly to our patients. I hope to continue a close and successful working relationship with you.”
“We want to thank you for giving us amazing resources. They are very dedicated and hardworking and manages multiple projects with ease and with good quality. They recently worked on complicated macros along with their regular project assignments and did a very good job. Thanks again. Please give us similar resources down the line.”
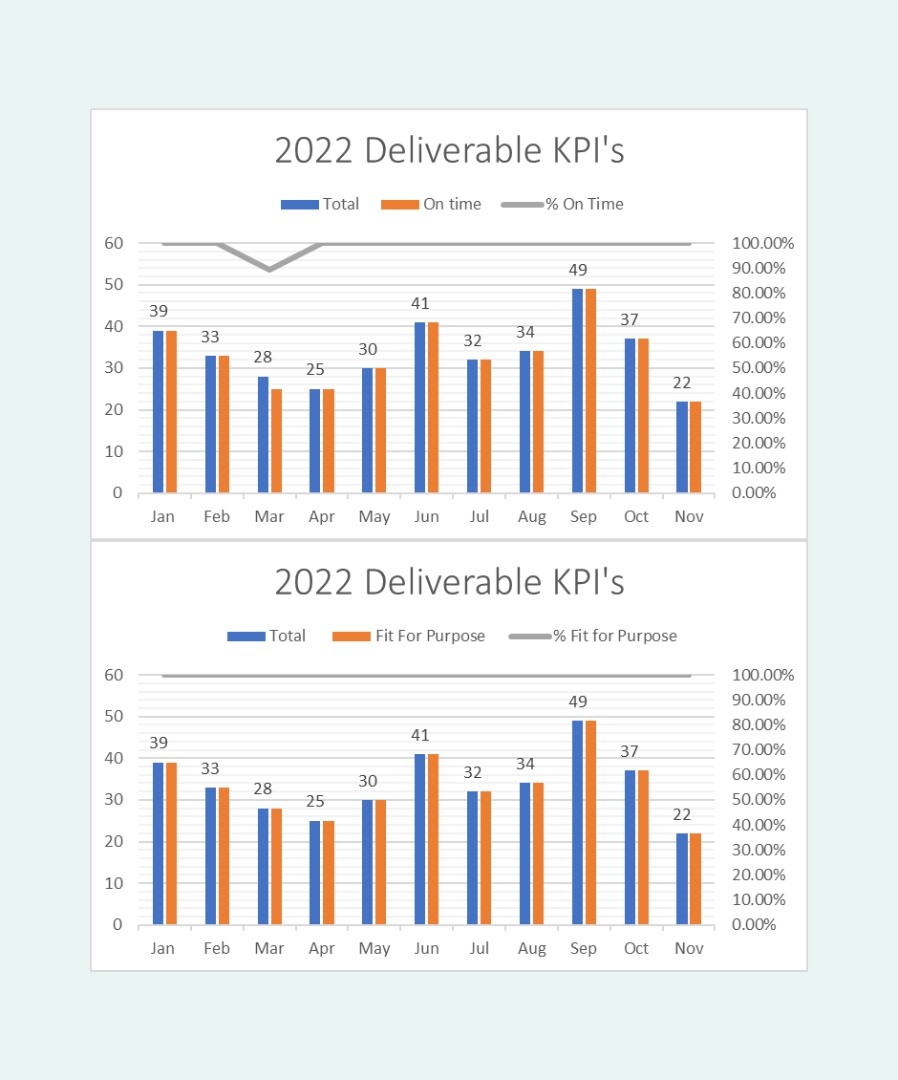
Visual Insights and Data-Driven Results
Quanticate’s contributions significantly improved the study's timelines and efficiency. Our programming team's ability to streamline data processing reduced the time required for regulatory submissions, directly impacting the speed at which the vaccine was authorised.
Our “first-time right” approach to quality also ensured no time was wasted on rework. These achievements are evident in our year end KPI metrics for 2022 when these successful COVID studies were completed.
Ready to achieve similar success with your clinical studies? Partner with Quanticate to accelerate your timelines, enhance data accuracy, and ensure regulatory success. Contact us today for a consultation and discover how we can support your project from start to finish.
Key Challenges and Lessons Learned
The primary challenge was the intense time pressure combined with the need for absolute accuracy in all deliverables. The team learned the importance of maintaining clear communication with the sponsor, which was crucial in managing expectations and ensuring alignment among all stakeholders throughout the project.
This experience also reinforced the value of having a flexible and resilient team capable of adapting to rapidly changing circumstances.
Conclusion
The extensive support Quanticate provided for these COVID studies, exemplifies our ability to deliver exceptional results under the most challenging conditions. By providing critical programming support, we played a key role in the successful development and authorisation of a vaccine that has saved countless lives. This case study showcases our expertise, dedication, and the tangible impact of our work, making it a compelling example of what we can achieve for future clients.
Global Reach
Quanticate’s involvement in this global initiative demonstrated our capability to operate effectively under pressure in a project of worldwide significance. By leveraging our extensive global footprint, we provided seamless 24/7 support by strategically assigning teams across multiple time zones. This approach allowed us to maintain continuous progress on the project, ensuring that no time was lost and that deliverables were met promptly.
By combining expertise from different regions, we adapted quickly to challenges, and maintain the momentum needed for such a critical project. The success of this study not only reinforced our reputation, but also expanded our reach in supporting global clinical trials, particularly in emergency situations, showcasing our ability to deliver high-quality results on a global scale.
Need a global programming partner? Quanticate’s worldwide team is ready to support your clinical trial 24/7.
Request a Consultation
We are here to help you accelerate your timelines, enhance data accuracy, and ensure regulatory success in your next project.
Fill out the form to begin.