Remote Source Data Verification (RSDV)
Reduce your travel costs and increase the speed to detection of issues when you verify source data remotely.
What is RSDV?
Remote source data verification (rSDV) is where the verification of source data is done remotely, off-site via a centralized location and by a specialist team. It is possible for multiple site’s source data to be checked at a single centralization location instead of going to a particular site. This introduces us to the concept of centralized monitoring.
When centralized monitoring and an rSDV approach is considered, the focus is usually on certain sites such as; high recruiting sites, low compliance sites, and sites with predicted or previous protocol deviations/violations. Also it is common for centralized monitoring and rSDV to focus on data points related to primary end points, safety data and certain trial related processes.
Benefits of RSDV
With the challenges caused by the Covid-19 pandemic and the reaction of the regulatory authorities suggesting remote approaches and a more patient centric approach to studies, more and more trials are looking to remote technologies.
Spend less on CRA travel as you monitor clinical trials remotely. Your CRAs will be able to review study documents and source data from anywhere, at any time.
By conducting SDV remotely, you perform precluding steps to a risk-based approach to clinical trials with RBM and CSM techniques to improve data integrity.
Speed up the detection and resolve both ICF and CRF queries as you no longer have to wait for psychical source data verification and timely respond times between monitors, investigators and site personnel.
How to perform RSDV
Step 1
Site teams sharing redacted copies of trial related source documents with the centralized team/monitor, using mobile apps or other technologies like uploading scanned PDFs to validated portals with audit trails.
Step 2
Video review of records; site staff sharing their computer screen using a secure video conference application.
Step 3
Providing restricted remote access (read only) to trial participants’ electronic medical records (EMR).

Key features of our RSDV Application



21 CFR part 11 compliant

Identifying potential problem sites sooner


Key Features of our Centralized Monitoring Team
Experienced monitors with therapeutic, GCP & regulatory knowledge
Team led by experienced individuals in global conduct of clinical trials
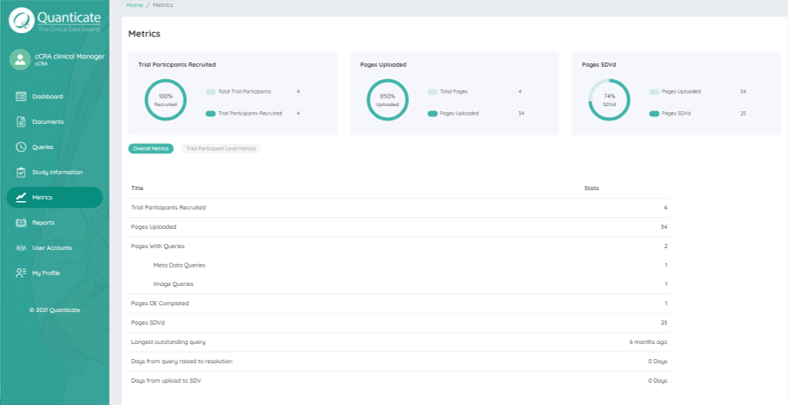
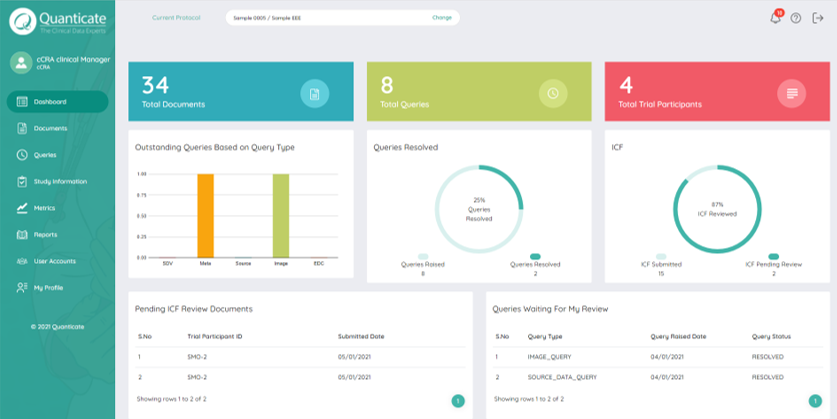
Enabling Risk Based Quality Management
Browse our additional remote monitoring resources
%20Guidelines.jpg?width=250&name=Statistical%20Monitoring%20in%20new%20ICH%20GCP%20E6(R2)%20Guidelines.jpg)
The Importance of Statistical Monitoring in the new ICH E6(R2) Addendum
Don’t let your data let you down
Bring your drugs to market with fast and reliable access to experts from one of the world’s largest global biometric Clinical Research Organizations.